Question #20be5
2 Answers
The latent heat of vaporization of ammonia is
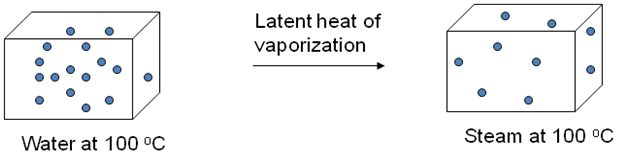
The latent heat of vaporization represents the energy you must supply, in the form of heat, to a substance to change it from liquid to vapor.
You're basically dealing with a phase change, during which you supply heat to the liquid ammonia to transform it to steam - at constant temperature.
Mathematically, the amount of heat required to vaporize a certain mass of a substance is given by
Plug in your data and solve for
If you round this to one sig fig, the number of sig figs given for 5 kg, the answer will be
Explanation:
The "Latent heat of vaporization" for a substance(in this case
We are told that
Hence,
But the question asks for "specific" latent heat,
so we divide by the mass,