Does methane have a sigma bond?
1 Answer
Apr 1, 2015
Yes it does, four sigma bonds actually.
Methane, or
The carbon atom is bonded to four hydrogen atoms through four single bonds; each single bond is also a sigma bond, which means that methane has a total of 4 sigma bonds.
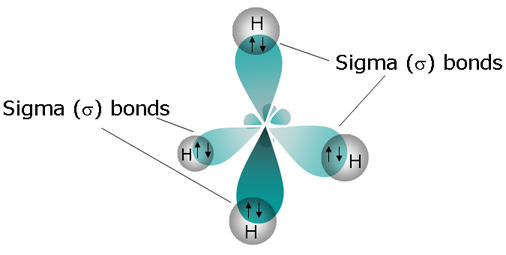
Each of carbon's four

