How to figure out whether a bond is more ionic than covalent?
2 Answers
For example:
NaCl and
NaCl is an ionic compound because the difference in electronegatity is 2.1 (Cl is 3.0 and Na is 0.9). If the result is bigger than (around) 1.8 we are talking about ionic compound.
If the electronegativity difference between the two elements is greater than or equal to 1.7, the bond is more ionic than covalent.
There are several formulas for calculating percent ionic character.
They give a fair correlation with ionic character as determined by dipole moments.
The formula proposed by Pauling is
Here's how it fits for a number of compounds.
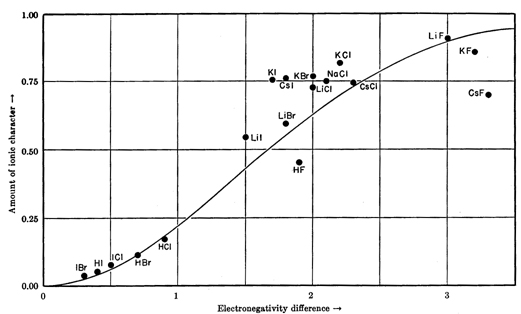
If
The predicted ionic character for HF is 55 %.
Here's a link to a downloadable Excel spreadsheet that calculates % ionic character.


