Question #51925
3 Answers
You need a Periodic Table. Find the element, then the atomic number listed.
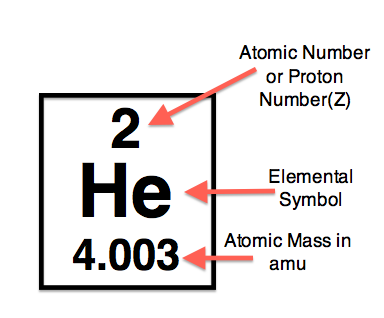
Your question is a bit vague. The atomic number will always be a whole number as it represents the number of protons in the nucleus. You could answer by saying "just count the protons" but that is not feasible unless you are looking at a diagram (as opposed to a real atom).
You may also be given the mass number (number of protons plus neutrons) and the number of neutrons. In this case the atomic number = number of protons = mass number - number neutrons.
Alternatively, if you know the number of electrons and the atom is electrically neutral, the number of protons = number of electrons since the charges are balanced.
You need a Periodic Table. Find the element, then the atomic number listed.

Your question is a bit vague. The atomic number will always be a whole number as it represents the number of protons in the nucleus. You could answer by saying "just count the protons" but that is not feasible unless you are looking at a diagram (as opposed to a real atom).
You may also be given the mass number (number of protons plus neutrons) and the number of neutrons. In this case the atomic number = number of protons = mass number - number neutrons.
Alternatively, if you know the number of electrons and the atom is electrically neutral, the number of protons = number of electrons since the charges are balanced.
You need a Periodic Table. Find the element, then the atomic number listed.

Your question is a bit vague. The atomic number will always be a whole number as it represents the number of protons in the nucleus. You could answer by saying "just count the protons" but that is not feasible unless you are looking at a diagram (as opposed to a real atom).
You may also be given the mass number (number of protons plus neutrons) and the number of neutrons. In this case the atomic number = number of protons = mass number - number neutrons.
Alternatively, if you know the number of electrons and the atom is electrically neutral, the number of protons = number of electrons since the charges are balanced.

