Question #0e196
1 Answer
Apr 15, 2015
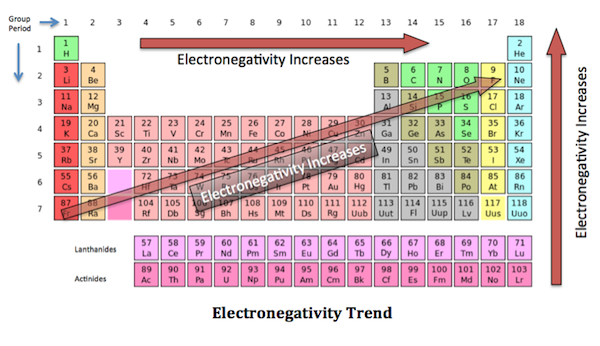
The more energy levels there are in an atom, the farther the electron clouds are to the nucleus and the easier it reacts with other elements. In contrast, electron clouds may be drawn closer to the nucleus due to increasing (+) charge in the nucleus, and that is why we see a electronegativity trend in the periodic table: left to right electronegativity increases, moving up in a group electronegativity also increases.