How do point mutations affect the synthesis of proteins?
1 Answer
To grasp this, you must firstly understand how the genetic code works. In the table below, you will see a list of amminoacids (that end in -ine; I will call them AA from now on) and for each AA, two or more nucleotide triplets (also called codon triplets) are associated. This means that each AA comes from the translation of a triplet in the genetic code, except for the "stop" codon (in yellow), which meaning is pretty clear! 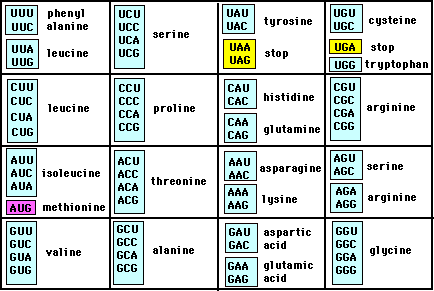
I suppose you already know that each protein is composed by a long chain, which links are the AA (the protein "primary" structure). Each AA has different chemical properties; some like water (they are hydrophilic), some don't (they are hydrophobic). Therefore, when the amino acid chain is in the watery environment of the cell, the hydrophobic parts will tend to "escape" from the direct contact of water molecules; the hydrophilic parts instead will tend to stay in contact with the water molecules.
This causes the protein to fold in substructures according to their configuration and preference to the exposition of water (secondary structure), and, finally, into their tri-dimensional final structures (the so-called tertiary structure), which is the final form (like Pokemon!) of the protein, the form that expresses the functionality of it, whether it is structural, functional, enzymatic etc.
Now, if we look at the table of the AA codons, we can see a pattern; let's take for example the amino AA (lower left).
Valine is an hydrophobic AA; it really despises water. We can see from the table that it can be formed by 4 codons, which differ only for the final base. This means that a change in the final base of the triplet codon will not change the translation of the AA valine; if you look around in the table, you will see this is quite common also for other amminoacids; all of them, except the tryptophan, are synthesized by at least two triplets, where, for each AA, the only difference is the last base of the codon. This peculiarity of the genetic code is called Degeneracy.
Now if a point mutation changes, let's say, GUG (the last triplet for valine) in GAG (which encodes for glutammic acid), the nature of the AA is radically changed; from the hydrophobic valine you will have the hydrophilic glutamic acid! This type of change can affect all the structure of the protein, altering its function.
These changes can be harmful for the organism, but sometimes, in particular conditions, a mutation like this can be advantageous as in the sickle cell disease, a condition that gives a slight advantage in areas affected from Malaria.
