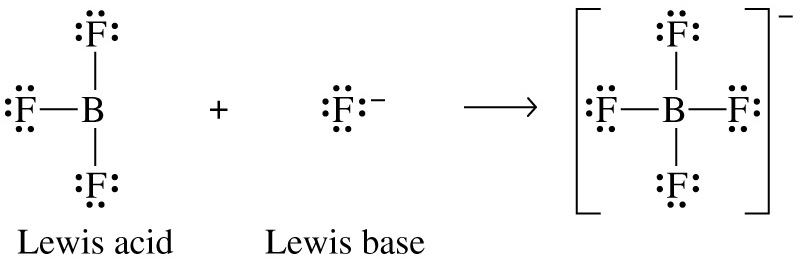What are the differences between Brønsted–Lowry model and the Lewis model for acids and bases?
1 Answer
Apr 17, 2015
The Arrhenius Theory of acids and bases
- Acids are substances that produce hydrogen ions in solution.
- Bases are substances that produce hydroxide ions in solution.
Neutralization happens because hydrogen ions and hydroxide ions react to produce water.
The Brønsted-Lowry Theory of acids and bases
- An acid is a proton (hydrogen ion) donor.
- A base is a proton (hydrogen ion) acceptor.
Very important - the Brønsted-Lowry theory does not go against the Arrhenius theory in any way, it just adds to it.

The Lewis Theory of acids and bases
This theory extends well beyond the things you normally think of as acids and bases.
- An acid is an electron pair acceptor.
- A base is an electron pair donor.