Question #75aa6
1 Answer
Apr 19, 2015
Sulfur dioxide, or
Two moles of sulfur will react with 1 mole of oxygen gas to produce two moles of sulfur trioxide.
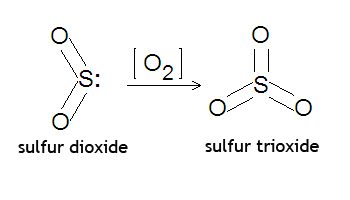
Read more about how you'd go about balancing this equation here:
http://socratic.org/questions/what-is-the-balanced-equation-of-so2-o2-so3

