Which one of the following compounds can be expected to have the highest lattice energy?

1 Answer
(1) MgO has the highest lattice energy.
Explanation:
The lattice energy depends on the attraction between the oppositely charged ions.
The force of attraction
The distance between the charges
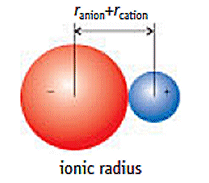
So the greatest attractions will be between ions that have the smallest radii.

And, since the attraction depends on the product of the charges, we look for ions with the greatest charge.
In both CaO and MgO, the charges are +2 and -2, so the choice is between these two oxides.
The smallest ions will be able to get closest to each other.
They will have the smallest distance between centres and will have the largest lattice energies.
The smallest ions are at the top of the Periodic Table.

