Question #21f00
1 Answer
Yes, that's a rough description of how the Lewis structure for formic acid,
Here's how you'd go about drawing the Lewis structure for this compound. The molecule has a total number of 18 valence electrons, 1 from each of the two hydrogen atoms, 4 from the carbon atom, and 6 from each of the two oxygen atoms.
Start by drawing connecting each of the five atoms through single bonds - this will use up 10 (2 for each single bond) of the 18 valence electrons and leave 8 more available.
Place these 8 valcence electrons as lone pairs on the oxygen atoms - 2 on the oxygen that is bonded to the carbon atom and to one of the hydrogens, and 2 on the oxygen that is bonded solely to the carbon.
Notice however that not all the atoms have complete octets. More specifically, the carbon atom only has only 6 valence electrons in the above structure - 2 for each single bond.
To solve this problem, and give carbon its complete octet, use one of the lone pairs of electrons present on oxygen to form a double bond between the two elements.
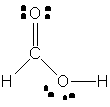
That will be the Lewis structure for formic acid,

