What is the likeliest m/z value for the base peak in the mass spectrum of 3-methylpentane?
1 Answer
May 6, 2015
The most likely value for the base peak in the mass spectrum of 3-methylpentane is at
The structure of 3-methylpentane is
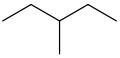
Mass spectroscopic fragmentation of hydrocarbons tends to occur at a branch.
So there are two possible fragmentation patterns:
- Loss of
CH3(M−15) .This will give a peak atm/z=71 , corresponding toCH3CH2CH+CH2CH3 . - Loss of
CH3CH2(M−29) . This will give a peak atm/z=57 , corresponding toCH3CH+CH2CH3 .
The peaks at 57 and 29 should be more intense than those at 71 and 15, because the ethyl cation (29) is more stable than the methyl cation (15).
The 2-butyl cation is more stable than the ethyl cation, so the base peak is probably at

