What are some examples of atomic spectra?
1 Answer
Every element has a unique atomic spectrum, and you can see it through a diffraction grating.
The atomic spectrum is a series of colored lines similar to the rainbow that a prism puts out when white light is sent through it. It's a series of colored lines, except their spacing, clarity, size, etc. are determined by the amount of energy put off.
Each element has its own spectrum because each element has its own electron configuration. You can show this using the equation
E(photon) = deltaE(atom)
This means that, when the atoms of the element are excited with electricity (google how a fluorescent lamp works for an explanation of this), they will give off light only in the same wavelength of energy as the energy put in. The energy used to excite the atom causes the electron to move down a few orbitals, closer to the nucleus. Because it is no longer at a high energy level, it will emit that same energy in the form of light at that specific wavelength.

Emission Spectrum of Hydrogen
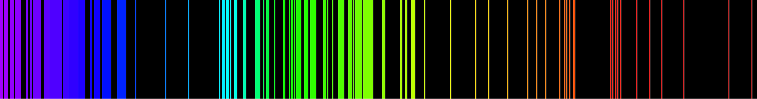
Emission Spectrum of Iron
Each slit represents one or more electrons that have released energy. Purple is released by highly excited electrons that move long distances. Red is released by (lowest frequency) less excited electrons.

