How do covalent compounds conduct electricity?
1 Answer
Covalent compounds conduct electricity by a quantum mechanical effect called quantum tunnelling.
Explanation:
Covalent compounds conduct electricity by a quantum mechanical effect called quantum tunnelling.
We say that covalent compounds do not conduct electricity.
But all substances conduct electricity to some extent.
Conductivity is a measure of the ability of a substance to pass an electrical current.
Electrons must be able to move through the substance for it to conduct electricity.
This is easy if the electrons are already delocalized, as in a metal or in an extended π system like graphite.
If all electrons are trapped in σ bonds, there are no delocalized electrons. The electrons are unable to move through the substance.
But a quantum mechanical effect called quantum tunnelling allows the electrons to tunnel through a barrier that they could not surmount in classical mechanics.
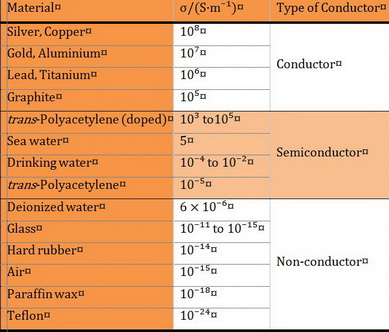
Conductivity (σ) is measured in units of siemens per metre (S/m).
It varies widely from
Semiconductors have conductivities in the range
Some polymers like polyacetylene can be made into good conductors.
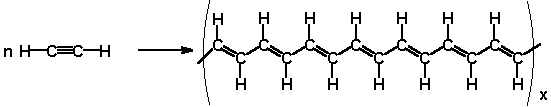
When small amounts of certain impurities are added, they create "holes" in the electronic structure.
When an electron jumps into a hole, a new hole is created, so charge can move a long distance.
This high conductivity upon doping helped to launch the field of organic conductive polymers.
Here are the conductivities of some substances.