Are solubilities of solids linear with temperature?
1 Answer
They don't have to be linear. Sometimes a solute is progressively easier to dissolve in a particular solvent as you add more solvent. It is like that for

This is kind of a special case. If you compare it with this picture:
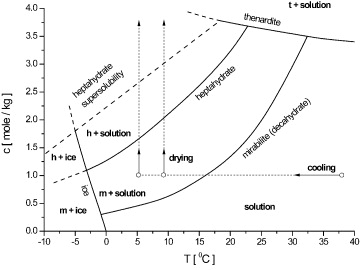
You can see that
So, you can also see that a solubility curve doesn't necessarily turn out to be linear or increasing. I haven't seen a curve that increases at a decreasing rate, but other than that, solubilities can decrease with increasing solvent, too, due to competing species.

