Question #129a3
1 Answer
May 19, 2015
Your solution has a volume of 6.0 L.
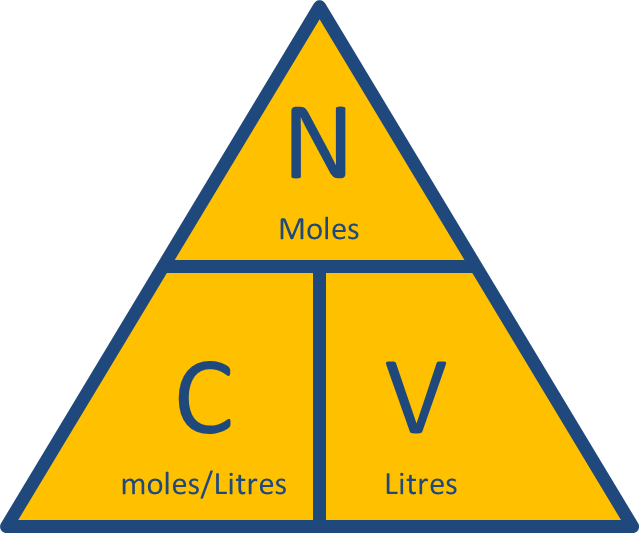
Molarity is defined as moles of solute per liters of solution. This means that if you have 1 mole of a solute in 1 L of solution you'll get a 1 M solution.
SInce you know both the number of moles of solute present, and the actual molarity of the solution, you can determine its volume by
In your case, the volume of the solution will be