What is the chemical formula for diamminedichloroethylenediaminecobalt(III) bromide? What are its nine isomers?
2 Answers
The chemical formula for diamminedichloroethylenediaminecobalt(III) bromide is
papadantonakis.com/images/1/1a/Chem_118_in_class_Worksheet_Key.pdf
I found four isomers of the diamminedichloroethylenediaminecobalt(III) ion on another pdf file at http://bchsapchemistry.wikispaces.com/file/detail/Chapter-24.Lectures+%2815%29.pdf . They are on page 5. You would just need to add the
I'm not completely sure about the isomers, so I'll just help you with the formula now and maybe update the answer tomorrow after I'm sure I have all the 9 isomers you mentioned.
So, you're dealing with a coodination complex, which consists of a central metal atom, in your case cobalt,
Notice that the name of the compound contains the cobalt (III) ion, or
The electron configuration of cobalt looks like this
Since cobalt is a transition metal, all the electrons located outside its noble gas core are considered to be valence electrons. The electron configuration of the
A low spin complex such as this one will have these 6 valence electrons paired in three of the five 3d-orbitals, and use the remaining two 3d-orbitals, its 4s-orbital, and the three 4p-orbitals to form 6
These hybrid orbitals will accomodate the electron pairs that come from the ligands.
So, you need to add six ligands to your central cobalt atom. According to the written formula, these ligands will be
- Two chlorine atoms - this comes from dichloro
- Two ammine functional groups - this comes from diammine
- One ethylenediamine (abbreviated en).
You only need one ethylenediamine because its structure looks like this
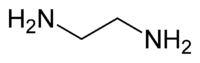
The two
So, if you put all this together you'll get
Here's how one isomer would look like


