How does the melting point of the substances change in a mixture?
1 Answer
This may be referring to colligative properties. If so, we can look at the following equation for chemical potentials:
This describes the chemical potential of a mixture in terms of the chemical potential of a pure substance that has a term corresponding to the added solute component.
The chemical potential can be thought of to be similar to energy states. The lower energy state tends to be desirable, so the lower chemical potential tends to be desirable.
According to the equation, if you dissolve a solute in a pure substance such as water, the mole fraction
Now, let's see what this has to do with melting/freezing points. Look at the following diagram:
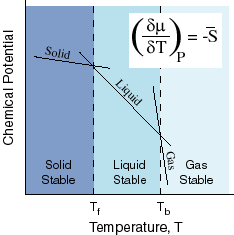
So, you see the line corresponding to the liquid, and also, you now know that the chemical potential for the liquid decreases when you dissolve a solute into a solvent. When that happens, every single point on the liquid line moves down, so try redrawing this, and then drawing a dashed line representing the liquid at a lower chemical potential (same slope), still intersecting the lines for the solid and gas (they stay still).
You can also see where the freezing point (
If you examine what you drew, the liquid-solid intersection point has moved left. That means the freezing point has lowered. This describes freezing point depression as a colligative property of a solute dissolved in a solvent.
That's why you put salt on icy roads (above

