Question #704b6
1 Answer
The formula unit for boron carbide is
Boron carbide forms a very complex crystal structure that can best be described as having a rhombohedral lattice unit that consists of
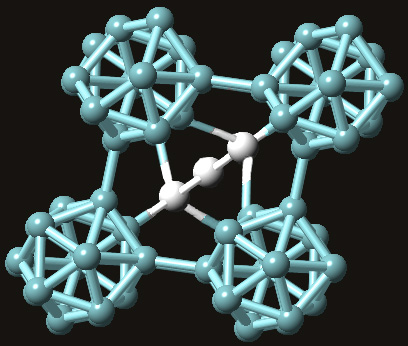
Depending on what combination of boron and carbon atoms make up that three-atom chain, you can have different formula units for boron carbide. This three-atom chain is usually believed to consists of two carbon atoms that flank a boron atom.
As a consequesnce of this arrangement of atoms, the formula unit could be given as
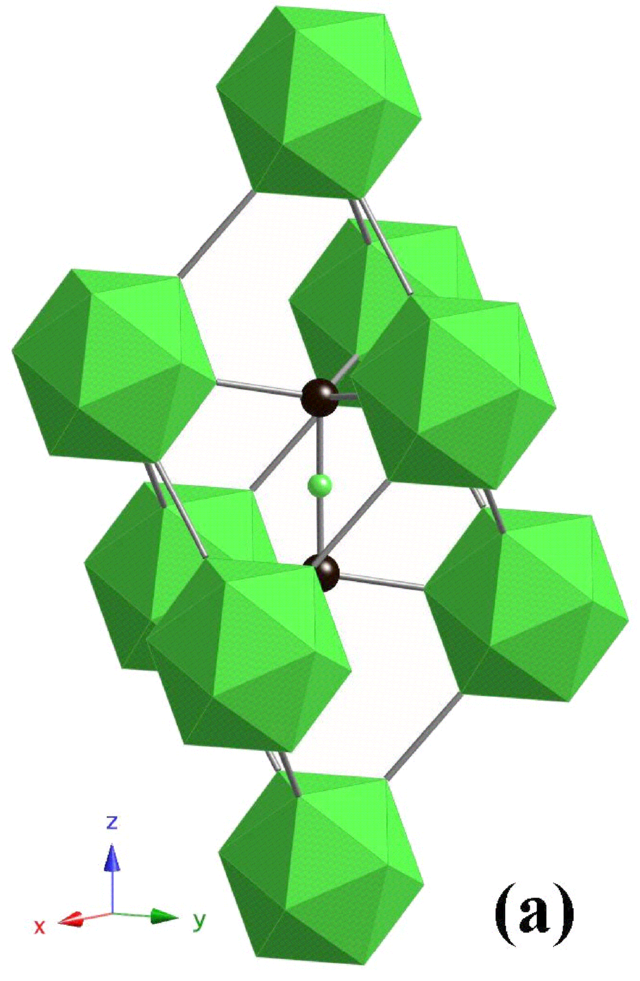
If you get two boron atoms flanking a carbon atom in the three-atom chain, you'll see the formula unit given as
A more conservative approach is to actually use
So, as a conclusion, the formula unit for boron carbide is usually given as

