Question #dd8a7
1 Answer
The iodine cyanide molecule,
If you take into account the fact that carbon is the central atom, the only Lewis structure that would give all the three atoms complete octets without any formal charges would look like this
The molecule has a total of 8 non-bonding electrons, distributed as 3 lone pairs on iodine and 1 lone pair on nitrogen, and 8 bonding electrons, distributed in 1 single bond and 1 triple bond.
All the 16 valence electrons are accounted for and all the have have complete octets.
Now for the phosphate ion,
Start by placing phosphorus as the central atom, and bonding it to the four oxygen atoms via single bonds. This will account for 8 of the 32 valence electrons, the remaining 24 valence electrons being distributed as lone pairs on the oxygen atoms.
Notice that all the atoms have complete octets, but that you have significant charge separation, which reduces the stability of the structure.
Since phosphorus is a period 3 element, it can actually have an expanded octet, i.e. accomodate more than 8 electrons in its outermost shell. This means that you form a double bond to one of the oxygen atoms in order to reduce the separation of charge.
As a result, you'll get a series of resonance structures that will look like this
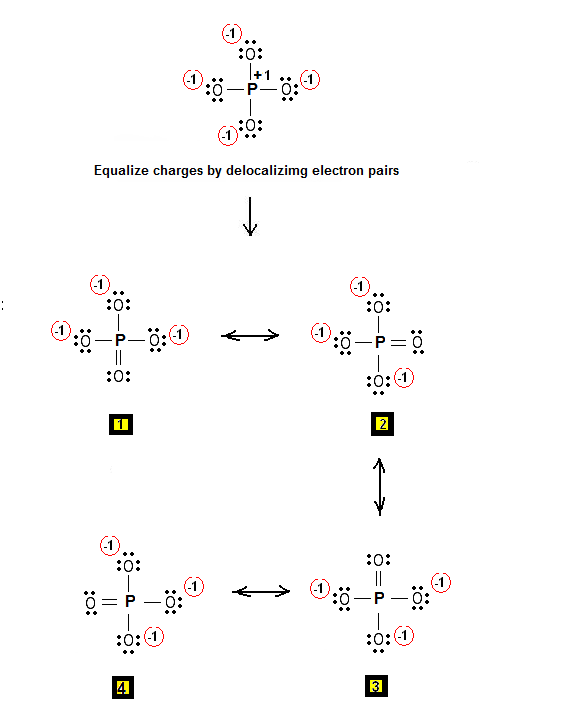
The actual Lewis structure of the phosphate ion is a hybrid of all these structures.

