Question #431cd
1 Answer
Before starting your analysis, keep in mind that a higher boiling point implies the presence of stronger intermolecular forces.
#MgCl_2# and#NF_3#
Magnesium chloride is an ionic compound, which implies that it is formed by the very strong electrostatic attractions that exist between positively and negatively charged ions.
In this case, the magnesium cations,
By comparison, nitrogen trifluoride is a covalent compound in which the atoms are held together by covalent bonds. Its electron geometry looks like this
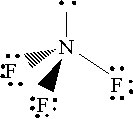
Since fluorine is more electronegative than nitrogen, the three
This molecule will exhibit dipole-dipole interactions, which are considerably weaker than ionic bonds. Furthermore, the strength of the dipole moment will be reduced because of the presence of the lone pair of electrons on the nitrogen atom.
Therefore,
#CH_3NH_2# and#CH_3F#
Both methylamine,
Hydrogen bonds ar a particular case of dipole-dipole interactions in which you have hydrogen bonded directly to one of the three most electronegative elements,
Hydrogen bonds are the strongest type of intermolecular bonds, so based on this alone, you can say that methylamine will have a higher boiling point than fluoromethane.
SIDE NOTE All the molecules exhibit weak London dispersion forces, but their importance is shadowed by the stronger dipole-dipole interactions and hydrogen bonds present.

