Question #f4b4c
1 Answer
In nature when we are talking about structure of compounds, we have limited set of options as far as elements in the periodic table are concerned. And when we are talking about biomolecules we have fewer choices, as not all elements are found to be involved in their structure.
Now, these few elements can connect with each other in infinitely different type of combinations to give rise to distinct compounds with unique physical and chemical properties. A simple analogy would be tangram puzzle/lego blocks which we can use to design different shapes and figures although the building blocks are same in all of them.
Carbohydrates are a class of biomolecules comprising of the elements Carbon (C), Hydrogen (H) and Oxygen (O) with a generic empirical formula of
They may be simple sugars or monosaccharides, which can combine with each other to give rise to complex carbohydrates like oligosaccharides (combination of 2-10 simple sugars) and polysaccharides (>10 simple sugars) .
 http://www.scientificpsychic.com/fitness/carbohydrates.html
http://www.scientificpsychic.com/fitness/carbohydrates.html
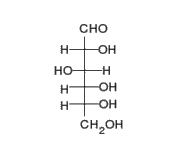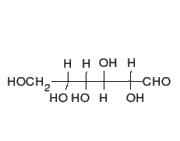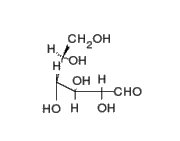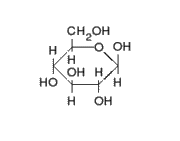
This is the structure of Glucose, the most widely available sugar in the living world with empirical formula of
Clearly as we can see from the two structures, there is no -COOH functionality in either ring or chain form.
There are many other compounds comprising of these 3 elements and not necessarily do they contain a carboxylate (-COOH) functionality. One common biomolecule would be cholesterol having a molecular formula of
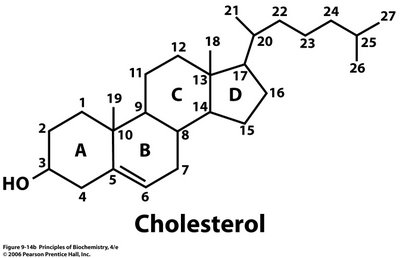 http://sandwalk.blogspot.in/2006/11/recording-lectures.html
http://sandwalk.blogspot.in/2006/11/recording-lectures.html

