Does #"C"_2# exist, and if so, describe its electron distribution?
1 Answer
Actually, diatomic carbon, or
This article from the literature discusses it:
http://pubs.acs.org/doi/abs/10.1021/ja00194a042
(You can view that in full if you have an ACS subscription.)
You can also prove this by constructing carbon's molecular orbital diagram.
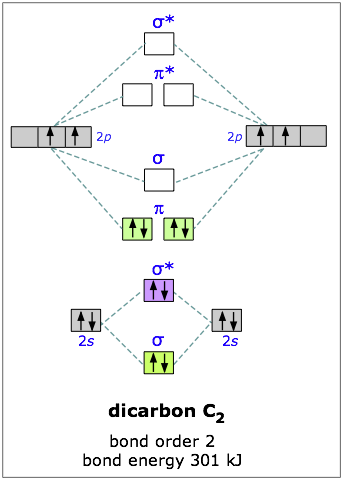
The
The diatomic carbon molecule has a bond order equal to
This suggests that the two carbon atoms are bonded via a double bond. However, as you can see, it's not a "classical double bond", meaning that it is not comprised of a sigma and a pi bond
If you go by this explanation, the Lewis structure of diatomic carbon looks like:
Those lone pairs of electrons are highly reactive, which is why diatomic carbon can only exist at temperatures close to
The literature article above describes how computational chemistry was able to predict the structure of
The ground-state singlet would look like a diradical
And the ground-state triplet would have two electrons in carbon 1's bonding
(the Lewis structure of the standard prediction of diatomic carbon above IS this structure, but doesn't indicate which lobes are being occupied)


