Question #e52b8
1 Answer
The DDT would be found in the fat, because both DDT and fat are mostly nonpolar molecules.
Explanation:
The structure of DDT is

DDT appears to have many polar
So, DDT is essentially a nonpolar molecule.
We know that Like dissolves like. That is, nonpolar solutes dissolve in nonpolar solvents.
Let's look at the structures of the three milk components and decide on their polarities.
The structure of a typical milk carbohydrate is
It has many polar
Casein is a typical milk protein. Its structure is
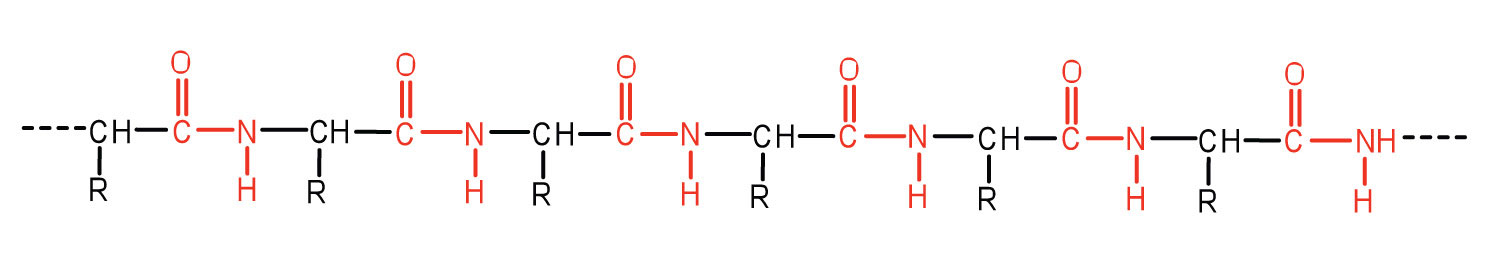
We see that it contains many polar
The structure of a typical milk fat is

We see that, except for a few
The fat is almost completely nonpolar. DDT will dissolve in the milk fats.

