What is the product when you put nitrobenzene and Tin in dilute HCl? How to predict this?
1 Answer
Jun 9, 2015
There is no product with dilute HCl but there is with concentrated HCl to give phenylamine
Explanation:
Tin and concentrated HCl form a suitable reducing agent to convert nitrobenzene to phenylamine:
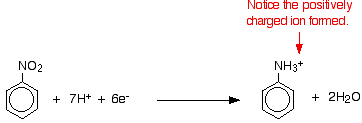
(chemguide.co.uk)
The electrons are provided by the oxidation of tin:
and:
To form the phenylamine base is added:
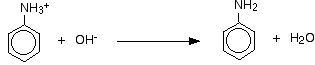
(chemguide.co.uk)

