Question #e87cd
1 Answer
Explanation:
A radical is a molecule or ion that has an unpaired electron. You can use the Lewis structures of the two compounds to see if either of them has an unpaired electron.
Start with carbon monoxide. The molecule has a total of 10 valence electrons, 4 from carbon and 6 from oxygen.
Its Lewis structure will looks like this
The triple bond that exists between carbon and oxygen accounts for 6 of the 10 valence electrons of the molecule, the rest being distributed as lone pairs on carbon and on oxygen, respectively.
As you can see, this molecule does not have an unpaired electron, so it cannot be radical.
Now take a look at
The hydroxide ion has a total of 8 valence electrons - 6 from oxygen, 1 from hydrogen, and 1 extra electron that gives it the negative.
Take a look at the Lewis structure of the hydroxide anion
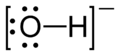
In order to get the neutral form, you're going to have to remove an electron from the ion. This electron can only come from a lone pair located on oxygen.
This will get you
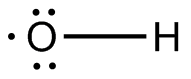
Now the ion has an unpaired electron, which means that it is a radical.
More often than not, you'll see

