Question #c8ac6
1 Answer
Explanation:
Phosphoric acid,
Silver chromate,
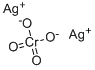
Since the chromate ion has a 2- charge, you need two 1+ silver ions to make a neutral compound.
Silver chromate thus contains two types of ions
- Positively charged ions:
#Ag^(+)# - Negatively charged ions
#CrO_4^(2-)#
The first compound you listed should actually be
Beryllium is located in period 2, group 2 of the periodic table, so it can lose two electrons from its outermost shell to form the beryllium cation,
The phosphate polyatomic ion,
Thus, beryllium phosphate contains
- Positively charged ions:
#Be^(2+)# - Negatively charged ions:
#PO_4^(3-)#

