Question #57f2d
1 Answer
Bromine water is simply a dilute solution of bromine.
Explanation:
Bromine water contains bromine molecules,
Here's how a sample of bromine water would look like

As you would image, the more dilute the solution is, the less dark its color will be.
Bromine water is usually used to test for the presence of alkenes. If you add an alkene to a sample of bromine water and shake the test tube, the solution will turn from light orange to colorless.
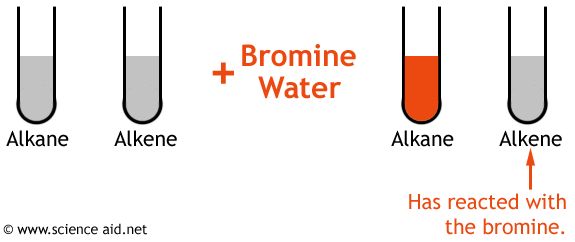
By comparison, alkanes cannot decolorize bromine water. The test is based on the halogenation reaction of alkenes, in which the halogen, in this case bromine, reacts with the carbon - carbon double bond.
This reaction breaks the double bond and turns the solution from light orange to colorless. Here's how two solutions, one containing an alkane and the other an alkene, look like after the addition of bromine water.

The test tube on the right contained an alkene, the one on the left an alkane.

