Question #d4d86
1 Answer
THe answer is d)
Explanation:
In order for two atoms that belong to different elements to be isobars, the must have the same mass number,
The mass number of an atom is equal to the sum of its protons and neutrons.
For any element, the mass number is located in the upper left of the chemical symbol, while the atomic number is located in the bottom left of the symbol.
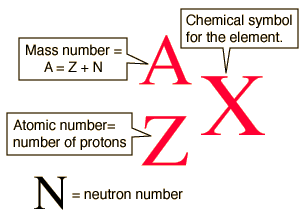 http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
So, all you have to do to determine which of those pairs is isobaric is to take a look at the number located in the upper left of the symbol.
For
The same can be said for
However, if you take a look at the atoms in the last pair, you'll notice that their mass numbers are indeed equal, which makes them isobaric.


