What type of reaction can produce insoluble salts?
1 Answer
Jun 21, 2015
Insoluble salts can be produced by precipitation reactions, in which two aqueous solutions react to produce a precipitate (an insoluble salt).
Explanation:
Examples:
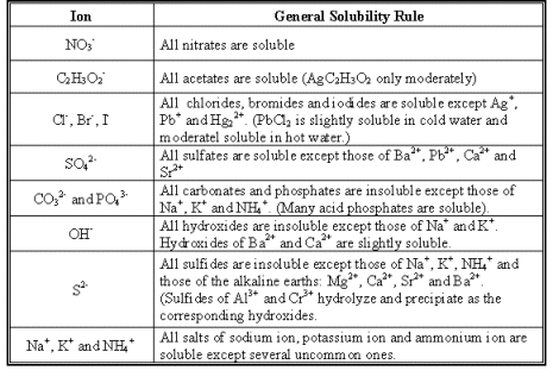
You can determine which compounds are insoluble by using a chart of solubility rules, such as the one below.