How many electrons are lost when methylbenzene is oxidised to benzoic acid ?
1 Answer
Jun 23, 2015
6 electrons are lost.
Explanation:
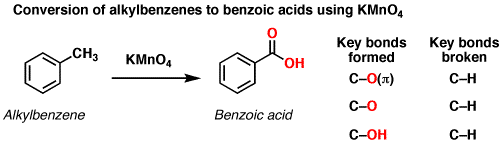
The oxidation number of carbon on the methyl group is -3.
When oxidised to benzoic acid it becomes +3.
This change releases 6 electrons.
The 1/2 equation becomes:
The electrons are taken in by the oxidising agent.

