At STP, what is the volume of #"2 mols"# of chlorine gas?
2 Answers
45.4 L.
Explanation:
The cool thing about STP conditions is that 1 mole of any ideal gas occupies exactly 22.7 L - this is known as the molar volume of a gas at STP.
STP conditions imply a temperature of 273.15 K and a pressure of 100 kPa. When tose conditions are met, 1 mole of any ideal gas will have a volume of 22.7 L.
So, if 1 mole occupies a volume of 22.7 L, 2 moles will occupy a volume twice as big.
Likewise, 0.5 moles will occupy half the volume 1 mole occupies.
SIDE NOTE Many online sources and textbooks still list the old STP conditions of 273.15 K and 1 atm. Under these conditions, the molar volume of a gas is actually 22.4 L.
If your teacher or textbook still uses that value, simply redo the calculation using 22.4 L instead of 22.7 L.
I'm going to assume the ideal case, but if you want to know a more accurate value, I'll show that below.
Using the Ideal Gas Law:
#PV = nRT#
At STP,
#V = (nRT)/P = [(2 cancel("mol"))(("0.083145 L"cdotcancel("bar"))/(cancel("mol"*"K")))(273.15 cancel("K"))]/(1 cancel("bar"))#
#=# #color(blue)("45.422 L")#
A more accurate answer would be
#P = (RT)/(barV - b) - a/(barV^2)#
In this case, we'd need to know the constants
#a = "6.343 bar/L"^2"mol"^2#
#b = "0.05422 L/mol"#
Now we need to solve for
#P = (RTbarV^2 - a(barV - b))/((barV - b)barV^2)#
(cross-multiply)
#PbarV^2(barV - b)= RTbarV^2 - abarV + ab#
(subtract through, multiply by denominator)
#PbarV^3 - bPbarV^2= RTbarV^2 - abarV + ab#
(distribute)
#PbarV^3 - bPbarV^2 - RTbarV^2 + abarV - ab = 0#
(move things around)
#PbarV^3 - (bP+RT)barV^2 + abarV - ab = 0#
(factor)
And now to solve this, one way I know of is to use the Newton-Raphson approximation method to approach the answer from above.
Let:
#P = color(green)(A) = color(green)("1 bar")#
#bP + RT = color(green)(B) = ("0.05422 L/mol")("1 bar") + ("0.083145 L"cdot"bar/mol"cdot"K")("273.15 K") = color(green)("22.76527675 L"cdot"bar/mol")#
#a = color(green)(C) = color(green)("6.343 bar/L"^2"mol"^2)#
#ab = color(green)(D) = "6.343 bar/L"^2"mol"^2 cdot "0.05422 L/mol" = color(green)("0.34391746 bar/L"cdot"mol"^3)#
#barV = X# in#"L/mol"#
Now we have:
#AX^3 - BX^2 + CX - D = 0#
What you can do is use the following formula for the Newton-Raphson method:
#color(darkblue)(X_"new" = X_"old" - (f(x))/(f'(x)))#
#f(x) = AX^3 - BX^2 + CX - D#
#f'(x) = 3AX^2 - 2BX + C#
Thus:
#color(darkred)(X_"new" = X_"old" - (AX^3 - BX^2 + CX - D)/(3AX^2 - 2BX + C))#
Now what you may want to do is store a number into each variable in this equation, because you'll have to keep recalling a long expression. The coefficients are given above.
For
Then, if you use a TI calculator, write this into your calculator to store variables:
#2->X#
#1 ->A#
etc.
and this to solve:
#(X - ((AX^3 - BX^2 + CX - D)/(3AX^2 - 2BX + C))) -> X#
and then press Enter until your answer stops changing.
-
Using
#X_"old" = 2# , I got#X_"new" = 0.2078358407# after 7 times pressing Enter. Knowing that, I would try something under#0.207# . -
Using
#X_"old" = 0.1# , I got#X_"new" = 0.0735975286# after 6 times pressing Enter. Having tried something near#1# and something below#1# , let's try something high. -
Using
#X_"old" = 20# , I got#X_"new" = 22.48384338# after 6 times pressing Enter.
This means then that the three answers I got are:
#"0.2078358407 L/mol"#
#"0.0735975286 L/mol"#
#"22.48384338 L/mol"#
The lowest answer corresponds to the volume of 1 mol of liquid chlorine. The highest answer is the volume of 1 mol of gaseous chlorine. The middle answer is nonphysical, so we're not going to use it. This looks something like this:
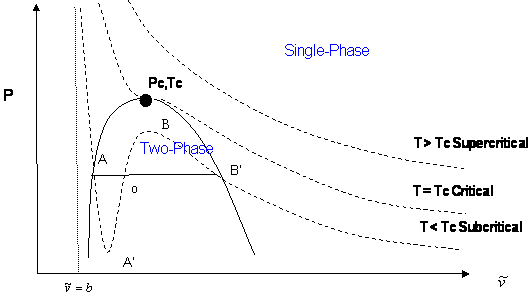
If you see the horizontal line below "Two-Phase", that is the intersection between the three answers. The liquid is on the left, and the gas is on the right.
Thus, to get the more realistic result for gaseous chlorine:
#"22.48384338 L/mol" * "2 mol" = 44.96768676 -> color(blue)("44.968 L")# compared to the ideal case of
#color(blue)("45.422 L")# .
Checking its compressibility factor:
#Z = (PbarV)/(RT) = ((1)(22.48384338))/((0.083145)(273.15)) = 0.989995#
Since


