Question #6e72b
1 Answer
Phosphorus trifluoride has 6 bonding electrons in sigma bonds and zero bonding electrons in pi bonds.
Explanation:
To be able to determine how many bonding electrons a molecule has in sigma bonds and pi bonds, take a look at its Lewis structure.
Every sigma bond contains two bonding electrons. Likewise, every pi bond contains two bonding electrons.
So, to determine how many of each you have, look for single bonds, which are actually sigma bonds, double bonds, which contain 1 sigma and 1 pi bond, and triple bonds, which contain 1 sigma and 2 pi bonds.
The Lewis structure of phosphorus trifluoride looks like this
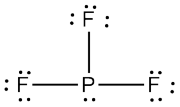
The central phosphorus atom is bonded to three fluorine atoms via single bonds, and has a lone pair of electrons attached.
The phosphorus atom is
As a result, phosphorus trifluoride has 2 sigma bonding electrons for every single bond present, for a total of 6 sigma bonding electrons, and zero pi bonding electrons, since it doesn't form any pi bonds.

