Question #071e5
1 Answer
Because a hydrogen molecule contains two hydrogen atoms.
Explanation:
A hydrogen molecule,
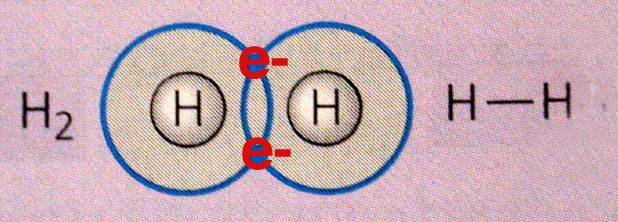 http://academic.brooklyn.cuny.edu/biology/bio4fv/page/covalent_bonds.html
http://academic.brooklyn.cuny.edu/biology/bio4fv/page/covalent_bonds.html
If a hydrogen molecule contains two hydrogen atoms, then its mass will be twice as big as that of an individual hydrogen atom.
A mole simply represents a collection or molecules or atoms. A substance's molar mass tells you how much one mole of that substance weighs.
In your case, one mole of hydrogen molecules will be twice as heavy as one mole of hydrogen atoms simply because each individual
So, one mole of hydrogen atoms has a mass of
2×1.00794 g = 2.01588 g

