What is the electron configuration of salt?
1 Answer
Assuming you mean
The electron configuration of
#1s^2 2s^2 2p^6 3s^1#
#= [Ne] 3s^1#
That for
#1s^2 2s^2 2p^6 3s^2 3p^5#
#= [Ne] 3s^2 3p^5#
If you mean them to be bonded, read on... The resultant VALENCE electron configuration is:
#(sigma_(3s, "nb"))^2 (sigma_(3p_z))^2 (pi_(3p_x))^2 (pi_(3p_y))^2#
DISCLAIMER: LONG ANSWER!
This is the MO diagram for NaCl (couldn't find a better one):
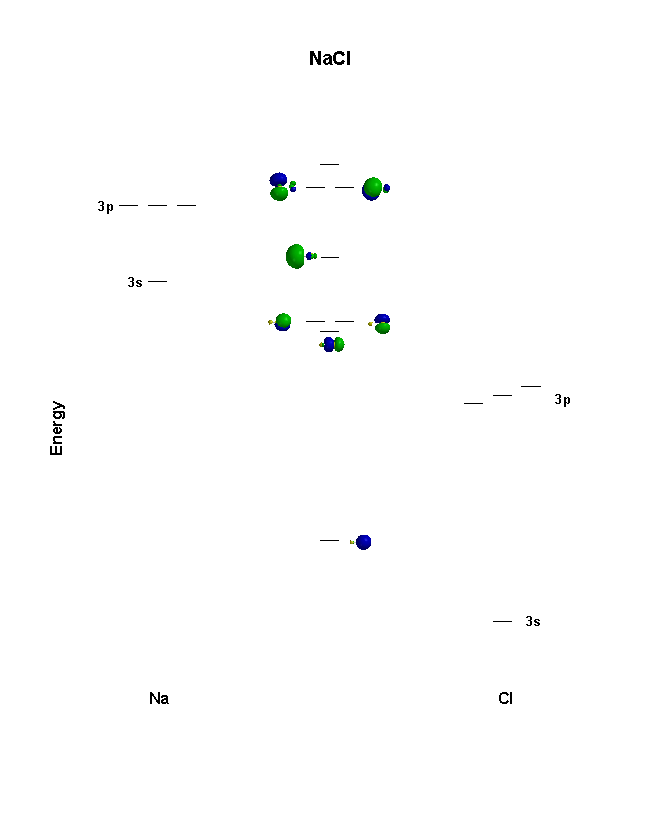
Writing the configuration for them when bonded would require you to know what molecular orbitals (MOs) are made from what atomic orbitals (AOs) when these two atoms bond.
To figure this out, you would have to construct or have an accurate MO diagram. Let
-
Two
#ns# orbitals combine to form one#sigma_(ns)# and one#sigma_(ns)^"*"# bonding and antibonding molecular orbitals, respectively. -
Two
#np_(x)# and#np_(y)# orbitals combine to form a#pi_(np_x)# and#pi_(np_y)# , and one#pi_(np_x)^"*"# and#pi_(np_y)^"*"# bonding and antibonding molecular orbitals, respectively. -
Two
#np_z# orbitals combine to form one#sigma_(np_z)# and one#sigma_(np_z)^"*"# bonding and antibonding molecular orbitals, respectively.
Given the above MO diagram, the configuration will contain the total number of electrons given by
#sigma_(1s)# ,#sigma_(1s)^"*"# #sigma_(2s)# ,#sigma_(2s)^"*"# #pi_(2p_x)# ,#pi_(2p_x)^"*"# #pi_(2p_y)# ,#pi_(2p_y)^"*"# #sigma_(2p_z)# ,#sigma_(2p_z)^"*"#
which would have contained
So, the particular MO diagram depicted above would fill up with only
The valence electron configuration would then be:
Valence electron configuration -----
#color(blue)((sigma_(3s, "nb"))^2 (sigma_(3p_z))^2 (pi_(3p_x))^2 (pi_(3p_y))^2)#
Note that the
Furthermore, the molecular orbital depictions have the

