The product of reacting an alkene with #"Br"_2(aq)# is #"C"_2"H"_4"BrOH"#. What is its name? Why is the product NOT a geminal dibromide?
Shouldn't an alcohol form? My teacher says there is no possibility of forming an alcohol.
Shouldn't an alcohol form? My teacher says there is no possibility of forming an alcohol.
3 Answers
You're teacher is quite correct, and there is no possibility of forming of alcohol (i.e. purely
Explanation:
Ethylene,
On the other hand, molecular bromine is polarizable, and can be represented as
The highlighted carbon is now cationic, and will react with any nucleophile present: this could be water, or cyanide, or bromide anion, whatever is in solution. Since we used
If, however, the olefin was treated with
Explanation:
The
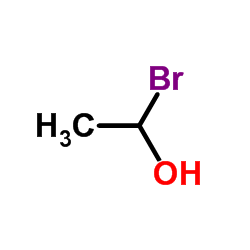
The following image is the structural formula for 1-bromoethanol.
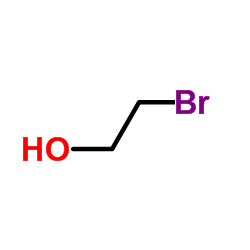
The following image is the structural formula for 2-bromoethanol.
Remember that aqueous means dissolved in water, which implies that water is present to react if its pKa is low enough (low pKa
It's quite true that bromine liquid reacting with ethene gives dibromoethane. That is an early mechanism you would have learned in the first few weeks of a first-year organic chemistry class.
However, water disrupts this mechanism in the middle. What happens is:
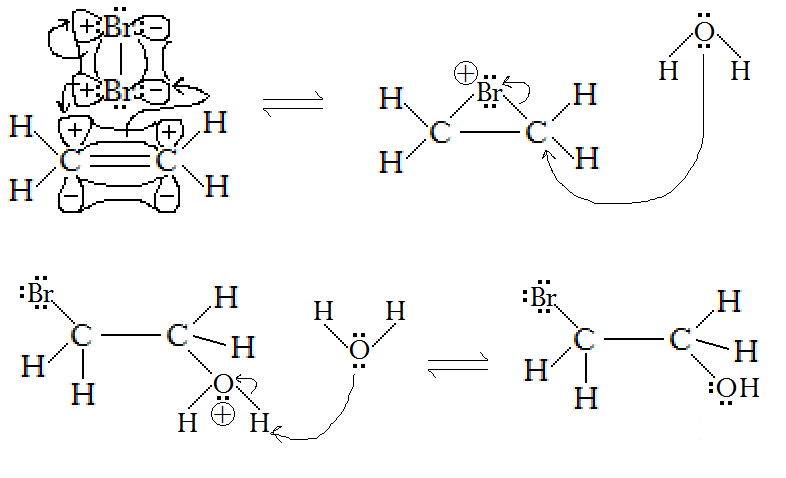
- In the first step, bromine makes the cyclopropane-analog bonds.
- Then the carbon centers of each
#"C"-"Br"# becomes electrophilic (electron-acceptor). Water, a stronger nucleophile than bromide, can attack either one (due to symmetry) from behind to bond and break one of the#"C"-"Br"# bonds, attaching in a#"trans-"# addition.
(normally, it would be the other bromide attacking from behind, not water)
- Water finishes the mechanism by deprotonating the attached water to form hydronium and bromide in solution.
So yes, there IS the possibility of forming an alcohol. In fact,



