Question #75490
1 Answer
Yes, covalent bonds can be polar and non-polar.
Explanation:
Covalent bonds (sharing of electrons) occur between two or more non-metals.
Polar covalent means there is a difference between the electronegativities of the two or more atoms that are bonding with each other, in other words there is an unequal sharing of the electrons involved in the bond, example of this would be HF, which is Hydrogen Flouride:
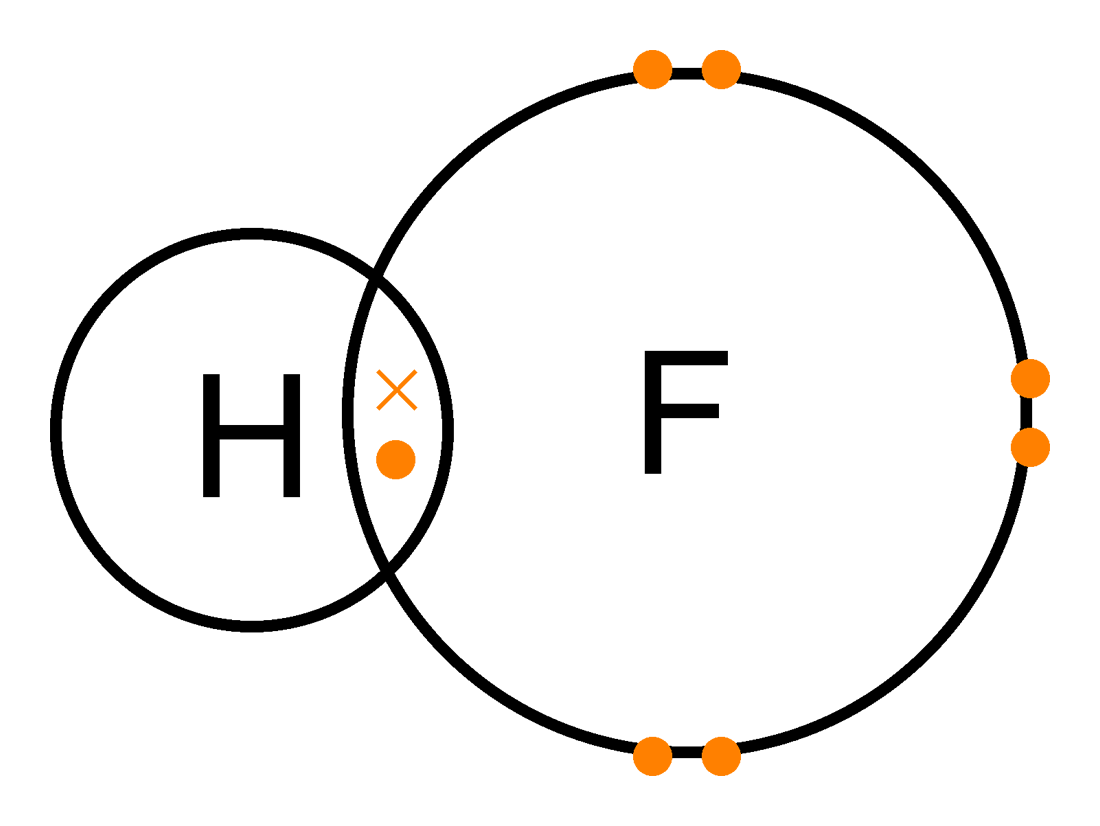
Image from: http://chem173pcb8-9a2011-2012.wikispaces.com/Problem+Set+12
In the case of non-polar covalent bonds there is an equal sharing of electrons involved in the bond, or in other words no electronegativity difference. This usually occurs in homo-diatomic molecules such as
Here's an image to differentiate the two:
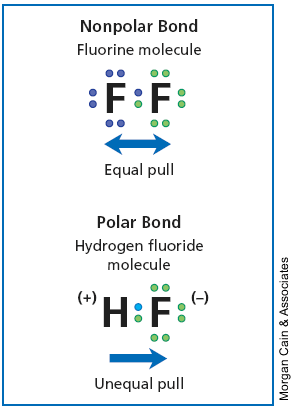
Image from: http://mycampus.nationalhighschool.com/doc/sc/Physical%20Science/iText/products/0-13-190327-6/ch5/ch5_s3_3.html
However if for instance you have
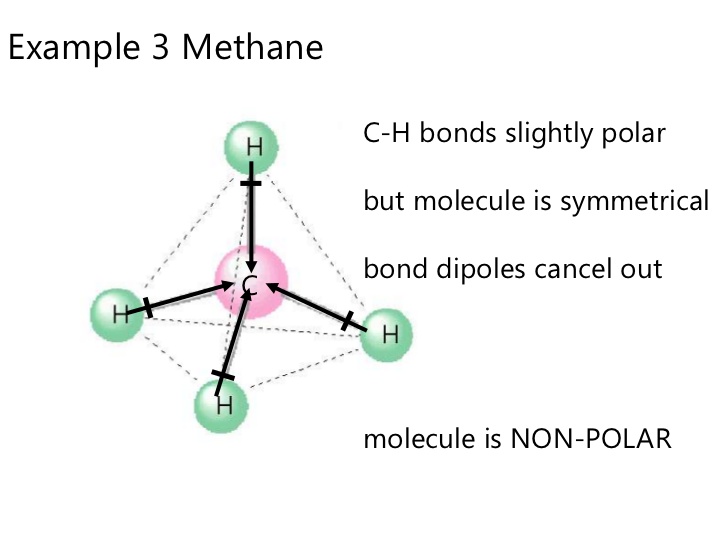
Image from: http://www.slideshare.net/pedagogics/2012-topic-42-polar-molecules
Hope I helped :)

