How does the amount of gas in a saturated solution change with pressure?
1 Answer
Sep 3, 2015
The amount of gas in a saturated solution is directly proportional to the pressure.
Explanation:
This is known as Henry's Law. It as often written as
where
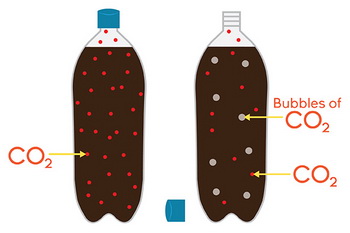
We see this effect in carbonated drinks.
Colas and root beer consist of flavoring and sugars added to a solution of carbon dioxide gas dissolved in water under pressure.
When the pressure is released, the solubility of the gas decreases, and bubbles of carbon dioxide escape from the liquid.
(from www.learner.org)

