What is the structure of #"Si"_3"N"_4#?
1 Answer
Sep 11, 2015
I would expect that the three silicon atoms are each in between a pair of nitrogen atoms. Silicon can make two double bonds, or one triple bond and one single bond.
Here is what this looks like:
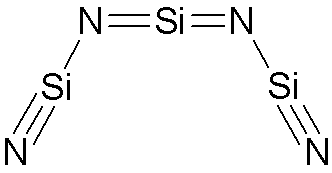
There are two lone valence electrons on the lewis structure at each nitrogen, and no lone valence electrons on each silicon.
The

