Question #19208
1 Answer
This reaction generates significant amounts of heat.
Explanation:
Sulfur trioxide reacts with water to form sulfuric acid,
#"SO"_(3(g]) + "H"_2"O"_text((l]) -> "H"_2"SO"_text(4(aq])#
The problem with this reaction is that it is extremely exothermic, meaning that it gives off very much heat.
Actually, this reaction gives off so much heat that it can actually vaporize the sulfuric acid solution, producing very dangerous sulfuric acid vapor.
Sulfuric acid vapor is very hard to condensate into liquid form, not to mention the fact that it is extremely corrosive, which makes it a dangerous pollutant.
So, to sum this up, the reaction between sulfur trioxide and water is very violent because it is highly exothermic. The heat produced by the reaction turns the product into very dangerous sulfuric acid vapor.
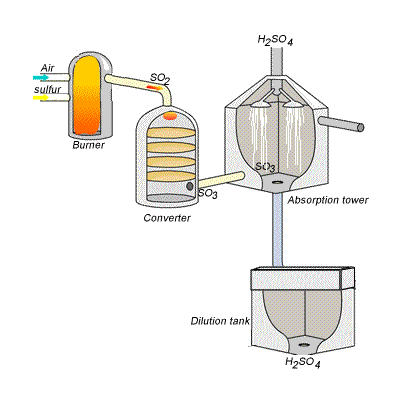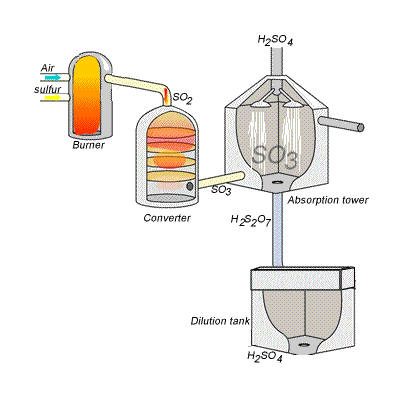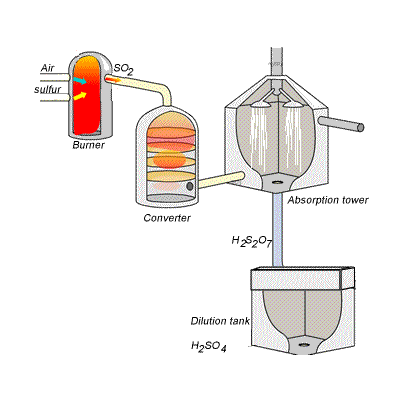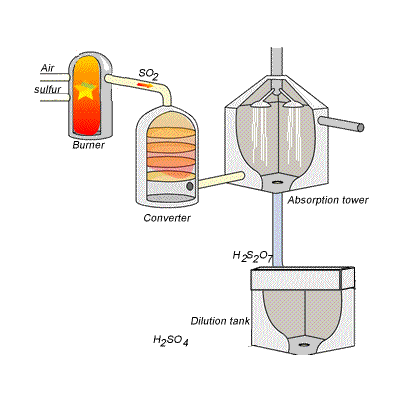
SIDE NOTE Sulfuric acid is actually produced by dissolving sulfur trioxide in a 98% solution of sulfuric acid - this is known as the contact process.
This reaction, which doesn't give off as much heat as the first one, produces oleum,