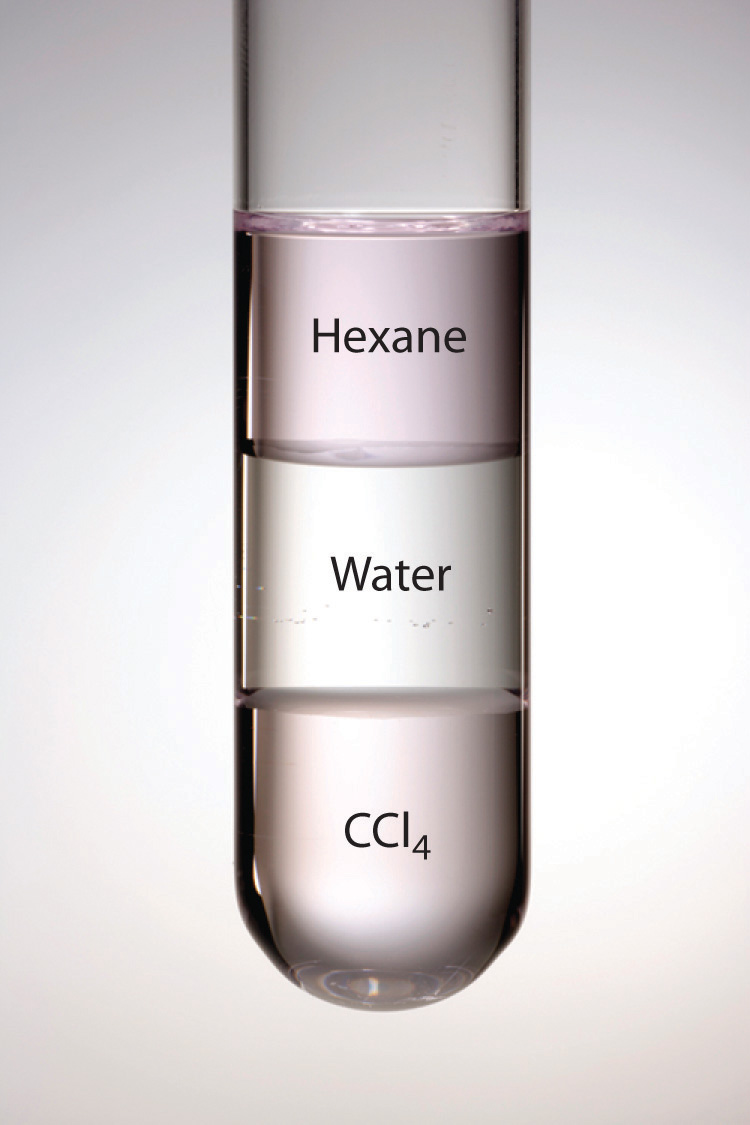
Why can't hexane dissolve in water?
1 Answer
Sep 20, 2015
Water and hexane are immiscible.
Explanation:
Water is a polar covalent substance and hexane is nonpolar. Like dissolve like. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Hexane can dissolve nonpolar substances, but not polar substances.