Question #25a3c
1 Answer
Q1) Group 2
Q2) Ca
Explanation:
Q1) The first four ioniation energies for an element are 738, 1450, 7730 and 10550 kJ/mol. Deduce the group number of the element (choices are 1,2,3,4).
The ionization energy increases when going from outside subshell to an inside subtle (s, p, d, f), and it increases dramatically when going from an outside shell to an inside shell (n=3 to n=2 or n=2 to n=1).
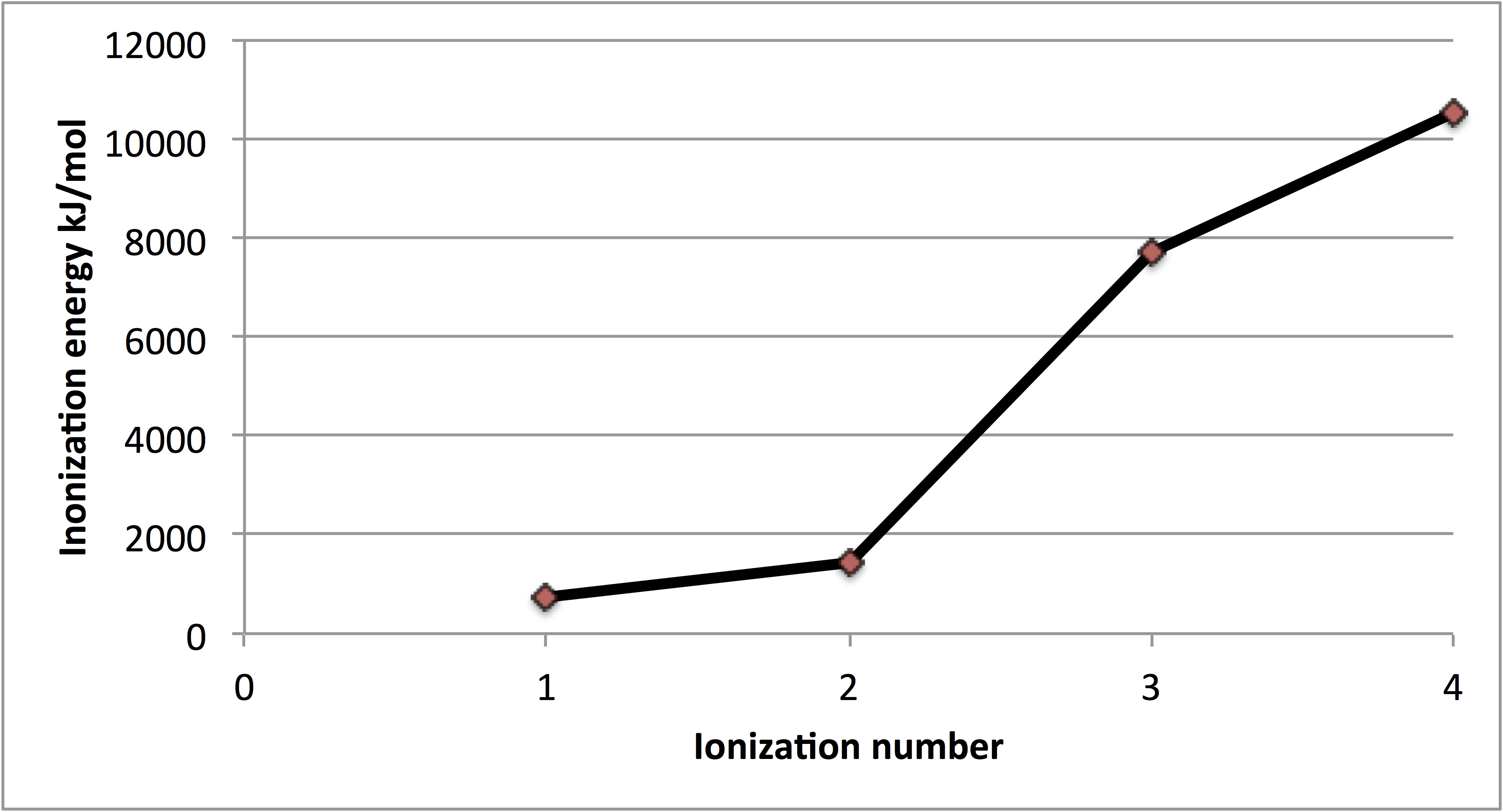
From the ionization energies we can see that there is an increase between 738 and 1450, which means that both electrons are taken from the same shell, however, for the third ionization energy 7730 kJ/mol, we can observe a sudden increase which implies a change of the shell level.
Having said that, it means that there was only two electrons on the outer shell, which means two valence electrons, and therefore the element belongs to group 2.
Q2) IE=590, 1145, 4912, 6491. Identify the element (choices are K,Ca,S,Cl) Please explain if possible?

The same explanation applies to this question. You can see the jump (sudden increase) between IE2 and IE3 which implies a change of the shell.
Therefore, two valence electrons, then the element is Ca.

