Is it possible to distinguish between first and second-order reactions based on a #[A]# vs. time graph?
1 Answer
If you are finding
The graphs would look like:
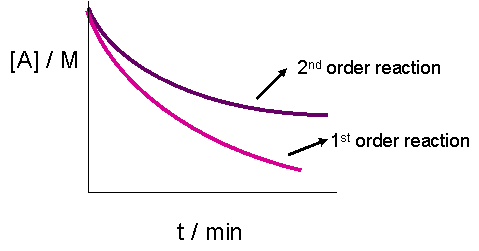
Without looking at both cases, it is impossible to tell by a graph. Even when superimposing both on the same graph, it may be hard to tell if it's really a second order vs. first order, or if it's just because a different mechanism occurred by accident that happened to give you that exact graph.
A zero order reaction is linear, though, so that you can distinguish from a first or second order reaction.
A better way to tell is by looking at a table. Let's suppose we have this (made-up) data:
Trial 1:
Trial 2:
Trial 3:
If you look at the trial concentrations I bolded, note the change in concentration of
- doubled concentration
- rate of reaction doubled
Thus, the reaction is first order in
- doubled concentration
- rate of reaction quadrupled
Thus, the reaction is second order in
For this reaction then, you can write:

