Question #63ac3
1 Answer
That depends on what you're comparing them to.
Explanation:
From what I can tell from the information you provided, this looks like a lab on osmosis and diffusion, perhaps part of a membrane transport lab?
If that is the case, then you probably have to determine whether or not two solutions are hypertonic, hypotonic, or isotonic, hence the terms relative solute concentration.
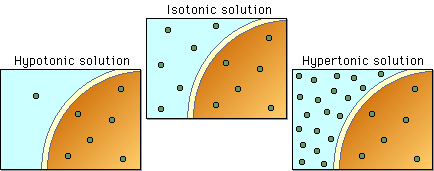
The idea here is that the concentrations of these solutions will tell you how much solute, in your case starch for one solution and glucose for the other, you get per 100 g of solution.
The percent concentration by mass of a solution is defined as
#"% m/m" = m_"solute"/m_"solution" xx 100#
In essence, a
The same goes for the
Now, to determine the relative solute concentration of two solutions, simply look at how much solute you get for an equal amount of the two solutions.
Now, let's say that you're comparing the
This time, the relative solute concentration of the
As a result, water will diffuse from the cell to the outside of the cell to balance the starch concentration
Let's sat that you're comparing the
This time, the relative solute concentration of the
As a result, water will diffuse inside the cell to balance the concentration of solute
Anyway, the idea is that the relative solute concentration of a solution can only be given by comparison to the relative solute concentration of another solution.

