Question #f91dc
1 Answer
Oct 6, 2015
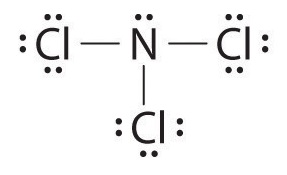
Nitrogen trichloride would have a trigonal pyrimidal shape and an AXE designation of
Explanation:
Nitrogen atoms have 5 valence electrons. The nitrogen atom has three unpaired electrons which will be shared with a chlorine atom (which has 7 valence electrons) to form a single bonds. This means the molecule has a central nitrogen bonded to three chlorine atoms each by a single covalent bond.
The other two valence of the nitrogen are a non-bonding pair and give the molecule its trigonal pyrimidal shape. This shape is predicted by using VSEPR theory.
Here are some videos to help with this concept:
Video from: Noel Pauller
Video from: Noel Pauller
Hope this helps!

