Is sodium carbonate insoluble in water?
1 Answer
Oct 7, 2015
No. Refer to the solubility rules below for the solubility of sodium carbonate
Explanation:
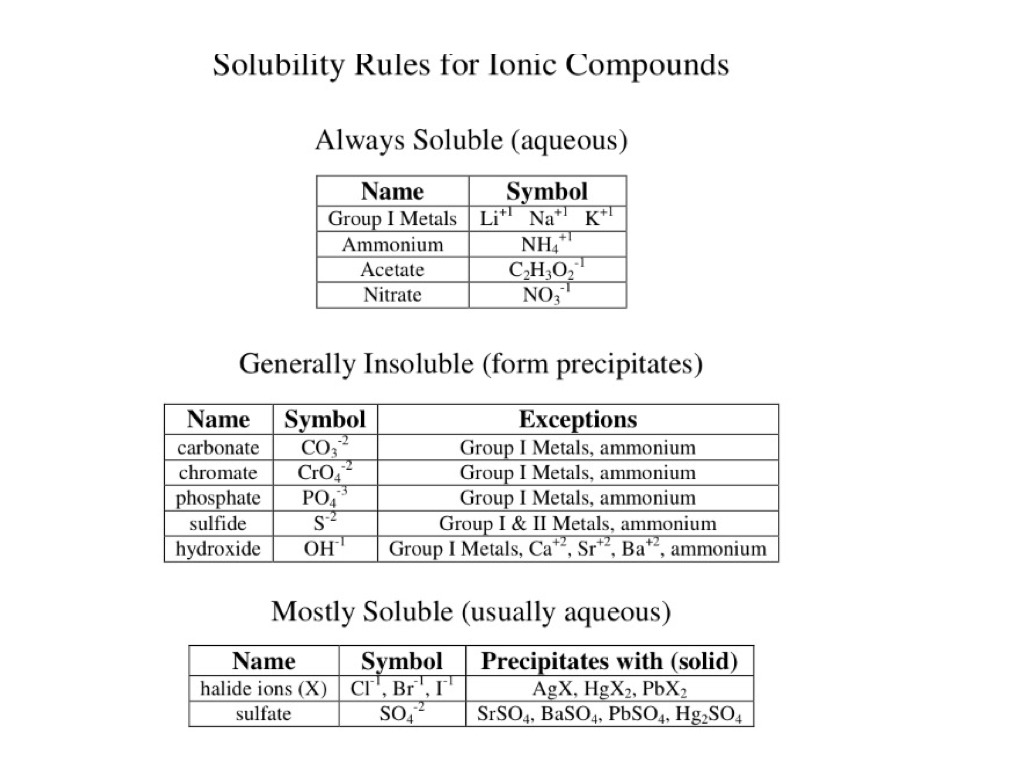
Since sodium is a group I metal, sodium carbonate is soluble and will not form a precipitate in any reaction.

