How would you represent potassium and bromine using an electron dot diagram? How about KBr?
1 Answer
Here's how that would look.
Explanation:
I'm not really sure if you're interested in the electron dot diagram of the potassium and bromine atoms, or of potassium bromide,
You can use this example to find the electron dot diagram of hydrogen bromide,
In order to draw an atom's electron dot diagram, you need to know two things
- the atom's chemical symbol
- the number of valence electrons it has
Start with potassium,
#["K"]: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1#
As you can see, potassium has one valence electron located on the fourth energy level in the 4s-orbital.
This means that its electron dot diagram will feature its chemical symbol and one dot, usually placed above the symbol.

Bromine,
#["Br"]: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 4p^5#
Bromine has seven valence electrons, all located on the fourth energy level in the 4s and 4p-obitals.
This means that its electron dot diagram will feature its chemical symbol surrounded by seven dots
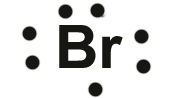
When potassium and bromine come together, the more electronegative bromine will snatch that solitary valence electron from the potassium atom.
This will lead to the formation of the potassium cation,
You draw the electron dot diagram by using the charges of the cation and anion.
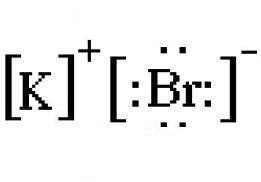
The resulting ions are placed in between square brackets. Notice that the valence electrons remain visible.

