How do enzymes increase reaction rates?
1 Answer
Enzymes increase reaction rates in a very similar way to regular catalysts, except they are much more effective.
Enzymes, unlike many catalysts:
- Give much higher reaction rates than regular catalysts.
- Act in mild conditions (
#37^oC# ,#"pH" = 7.4# ). - Are highly specific (sometimes stereospecific), which is one of the most important ways enzymes differ from normal catalysts.
- Are easy to regulate/control, such as by feedback inhibition or feedforward inhibition.
They provide an alternate reaction pathway to lower the Gibbs' free energy of the transition state,
This allows the reaction to proceed more quickly, but the enthalpy, entropy, and Gibbs' Free Energy of reaction are all the same as the uncatalyzed reaction.
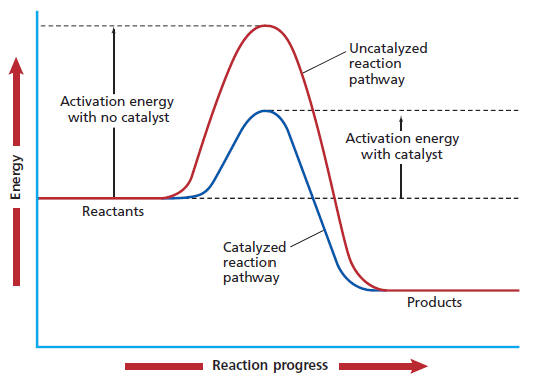
Their key virtues that facilitate such high reaction rates are:
-
Geometric specificity to the substrate binding site
Perfect shape to fit into binding site
-
Charge specificity to the substrate binding site
e.g. A
#(+)# sidechain binds at a binding site lined with#(-)# , etc.
Those together help the enzyme to achieve the proper orientation and to pick the proper location to which they can bind.
Some common ways in which catalysis can occur:
-
Acid/base catalysis
Proton transfer, similar to acid-catalyzed hydrations
-
Covalent catalysis
Bind to substrate while reaction occurs to alter mechanism, comes off later
-
Metal-ion catalysis
Something like
#"Fe"^(2+)# catalyzes the#"O"_2# binding capability of hemoglobin. Specifically for hemoglobin, when#"O"_2# binds to#"Fe"^(2+)# , the iron ion moves into the plane of the heme ring within hemoglobin, promoting the rest of the hemoglobin nearby to do the same thing to their structure, even without binding to#"O"_2# ; then binding to#"O"_2# becomes easier. -
Orientation catalysis
Binds to orient the reactants in the optimal way for a faster reaction rate
-
Preferential binding to the transition state
Binds specifically to the transition state and stabilizes it, facilitating the formation of the transition state and thus speeding up the reaction

