Question #7a5cd
1 Answer
Oct 17, 2015
You heat it!
Explanation:
Your goal with this reaction is to produce iron(II) sulfide,
#"Fe"_text((s]) + "S"_text((s]) stackrel(color(red)("heat")color(white)(xx))( -> ) "FeS"_text((s])#
You start by mixing iron and sulfur powder
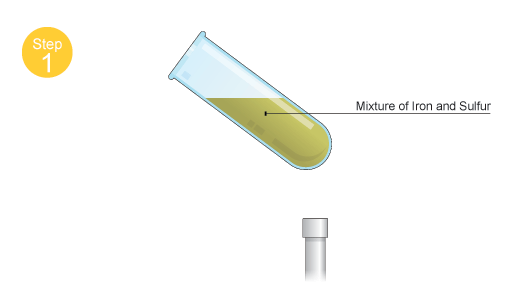
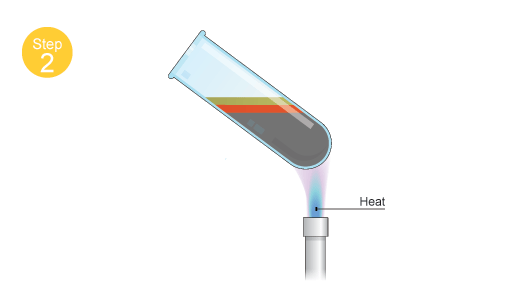
Once heat is provided to the mixture, the sulfur will melt and it will react with the iron. The reaction is exothermic, which means that iw will give off heat as it progresses.
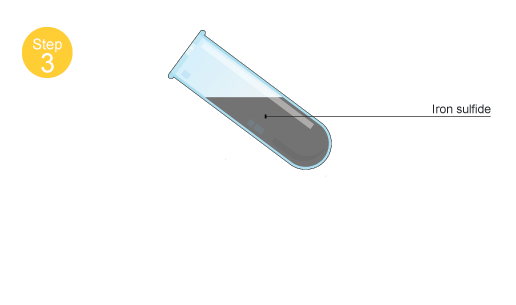
The reaction will form the black solid iron(II) sulfide,
Finally, a cool video of the reaction

