Question #072db
1 Answer
Oct 20, 2015
Here's what I got.
Explanation:
You know that your solution contains metal cations.
You also know that this solution will produce a precipitate, which is an insoluble solid, when mixed with a solution of sodium chloride,
Sodium chloride is a soluble ionic compound, which means that it will dissociate completely to form sodium cations,
This means that the pricipitate will be a halide, or, more specifically, a chloride.
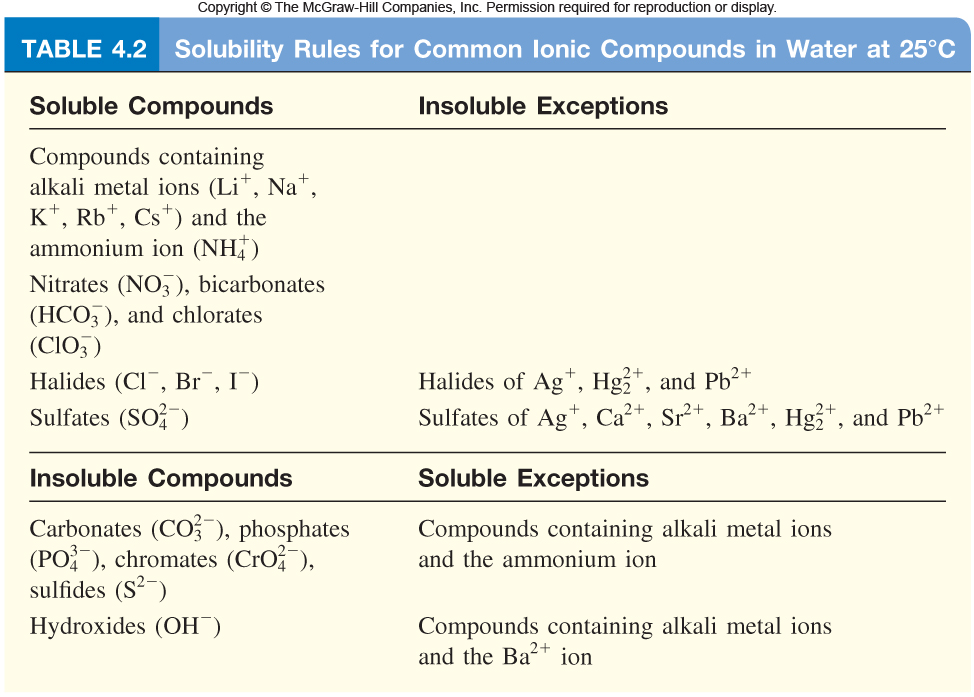
If you take a look at the solubility rules for chlorides, you'll notice that three common metal ions will produce insoluble solids with the chloride anion
- Lead(II) cation
#-># #"Pb"^(2+)# - Mercury(I) cation
#-># #"Hg"_2^(2+)# - Silver(I) cation
#-># #"Ag"^(+)#
So, the three ionic compounds that can precipitate out of solution are
- Silver chloride,
#"AgCl"#

- Mercury(I) chloride,
#"Hg"_2"Cl"_2#

- Lead(II) chloride,
#"PbCl"_2#


