Valency is a concept that is defined by the power of atom/s to combine with other atoms to form a substance or compound.
Electrovalency is the transfer of electrons from one atom to the other.
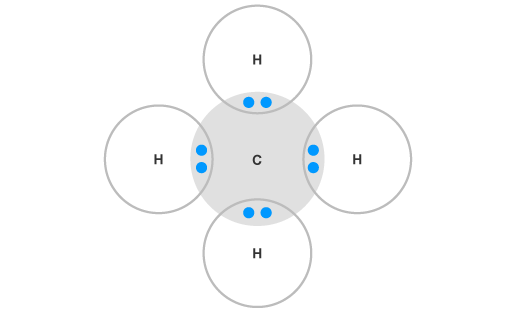
For example, carbon has 4 valence electrons which means it can, at most, form 4 single bonds with 4 other atoms (e.g. #CH_4#). This is valency.
On the other hand, in the molecule #NaF#, the Na atom willingly gives up its one electron to make the #Na^+# ion, which the fluorine atom willingly accepts to make the #F^-# ion. They both do this in their bid to mimic the electron configuration of the nearest noble gas. This is electrovalency.
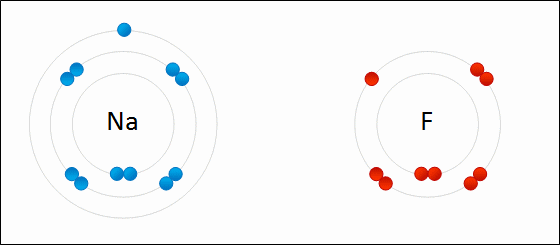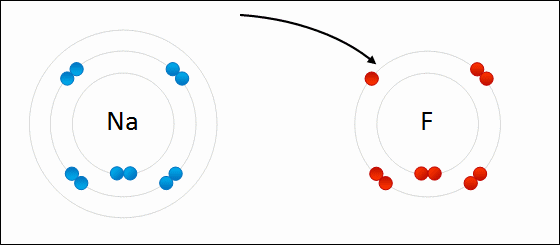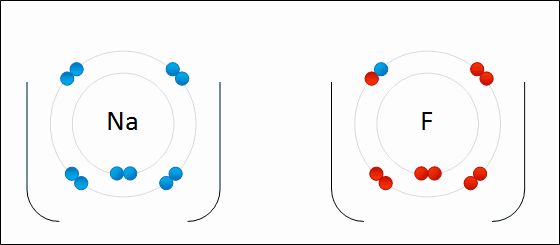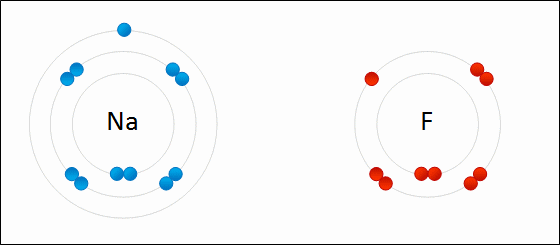
#Na^o#: #1s^2# #2s^2# #2p^6##3s^1#
#Na^+#: #1s^2# #2s^2# #2p^6# = same as #Ne# (atomic number 10)
#F^o#: #1s^2# #2s^2# #2p^5#
#F^-#: #1s^2# #2s^2# #2p^6# = same as #Ne# (atomic number 10)



